
Services
Testing Laboratory
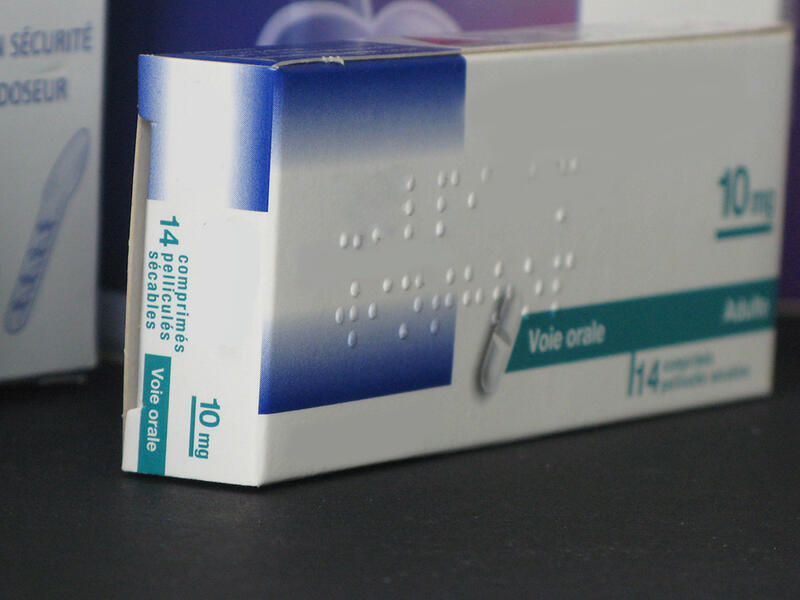
Verification of Braille marking
Measurement of Braille dot height
- Braille dot height measurement using a non-contact optical technique
- Quality control for folding carton converters and the pharmaceutical industry
- Testing in accordance with NF EN ISO 17351 Packaging - Braille on packaging for medicinal products
General informations
European Union directive 2004/27/EC made it compulsory to print the name and strength of medicinal products for human use in Braille on pharmaceutical packaging, for all new marketing authorisations granted as of 30 October 2005.This directive has been further strengthened by the ISO 17351 standard, which requires the height of the dots to be verified in order to ensure that the Braille labelling is clearly legible for visually-impaired users.
This need to guarantee and verify the quality of Braille marking primarily concerns manufacturers of folding boxboard cartons for pharmaceutical packaging and all the players in this sector. However, Braille labelling is also applied in other sectors such as over-the-counter pharmaceuticals, cosmetics, and food.
Technical Data and Achievements
In accordance with NF EN 15823, “Braille on packaging for medicinal products”
- Determination of Braille dot height, using a non-contact optical technique based on laser triangulation.
- The following data are supplied: height of each dot, average and standard deviation of the distribution of heights per carton, number of dots less than 0,1 mm high, number of dots less than 0,12 mm high.
- A 3D image of the Braille dots can also be provided.
 |
 |
|
Also to be seen
Centre of Excellence
Testing Laboratory
Laboratories